Daniel Engber?s three-part series ?The Mouse Trap: How One Rodent Rules the Lab? (published in Slate last November) has won the 2012 Communication Award for the online category. The award is conferred annually by the National Academy of Sciences, National Academy of Engineering, and Institute of Medicine with funding from the W.H. Keck Foundation to honor ?excellence in reporting and communicating science, engineering and medicine to the general public.??Engber's exploration of the risks of relying on mice for so much of our research brought an underreported scientific problem to light for lay readers. You can revisit the series below. undefined
Mark Mattson knows a lot about mice and rats. He's fed them; he's bred them; he's cut their heads open with a scalpel. Over a brilliant 25-year career in neuroscience?one that's made him a Laboratory Chief at the National Institute on Aging, a professor of neuroscience at Johns Hopkins, a consultant to Alzheimer's nonprofits, and a leading scholar of degenerative brain conditions?Mattson has completed more than 500 original, peer-reviewed studies, using something on the order of 20,000 laboratory rodents. He's investigated the progression and prevention of age-related diseases in rats and mice of every kind: black ones and brown ones; agoutis and albinos; juveniles and adults; males and females. Still, he never quite noticed how fat they were?how bloated and sedentary and sickly?until a Tuesday afternoon in February 2007. That's the day it occurred to him, while giving a lecture at Emory University in Atlanta, that his animals were nothing less (and nothing more) than lazy little butterballs. His animals and everyone else's, too.
Mattson was lecturing on a research program that he'd been conducting since 1995, on whether a strict diet can help ward off brain damage and disease. He'd generated some dramatic data to back up the theory: If you put a rat on a limited feeding schedule?depriving it of food every other day?and then blocked off one of its cerebral arteries to induce a stroke, its brain damage would be greatly reduced. The same held for mice that had been engineered to develop something like Parkinson's disease: Take away their food, and their brains stayed healthier.
How would these findings apply to humans, asked someone in the audience. Should people skip meals, too? At 5-foot-7 and 125 pounds, Mattson looks like a meal-skipper, and he is one. Instead of having breakfast or lunch, he takes all his food over a period of a few hours each evening?a bowl of steamed cabbage, a bit of salmon, maybe some yogurt. It's not unlike the regime that appears to protect his lab animals from cancer, stroke, and neurodegenerative disease. "Why do we eat three meals a day?" he asks me over the phone, not waiting for an answer. "From my research, it's more like a social thing than something with a basis in our biology."

Illustration by Rob Donnelly.
But Mattson wasn't so quick to prescribe his stern feeding schedule to the crowd in Atlanta. He had faith in his research on diet and the brain but was beginning to realize that it suffered from a major complication. It might well be the case that a mouse can be starved into good health?that a deprived and skinny brain is more robust than one that's well-fed. But there was another way to look at the data. Maybe it's not that limiting a mouse's food intake makes it healthy, he thought; it could be that not limiting a mouse's food makes it sick. Mattson's control animals?the rodents that were supposed to yield a normal response to stroke and Parkinson's?might have been overweight, and that would mean his baseline data were skewed.
"I began to realize that the ?control? animals used for research studies throughout the world are couch potatoes," he tells me. It's been shown that mice living under standard laboratory conditions eat more and grow bigger than their country cousins. At the National Institute on Aging, as at every major research center, the animals are grouped in plastic cages the size of large shoeboxes, topped with a wire lid and a food hopper that's never empty of pellets. This form of husbandry, known as ad libitum feeding, is cheap and convenient since animal technicians need only check the hoppers from time to time to make sure they haven?t run dry. Without toys or exercise wheels to distract them, the mice are left with nothing to do but eat and sleep?and then eat some more.
That such a lifestyle would make rodents unhealthy, and thus of limited use for research, may seem obvious, but the problem appears to be so flagrant and widespread that few scientists bother to consider it. Ad libitum feeding and lack of exercise are industry-standard for the massive rodent-breeding factories that ship out millions of lab mice and rats every year and fuel a $1.1-billion global business in living reagents for medical research. When Mattson made that point in Atlanta, and suggested that the control animals used in labs were sedentary and overweight as a rule, several in the audience gasped. His implication was clear: The basic tool of biomedicine?and its workhorse in the production of new drugs and other treatments?had been transformed into a shoddy, industrial product. Researchers in the United States and abroad were drawing the bulk of their conclusions about the nature of human disease?and about Nature itself?from an organism that's as divorced from its natural state as feedlot cattle or oven-stuffer chickens.
 Mark Mattson, National Institute on Aging
Mark Mattson, National Institute on AgingCourtesy of the National Institute on Aging.
Mattson isn't much of a doomsayer in conversation. "I realized that this information should be communicated more widely," he says without inflection, of that tumultuous afternoon in Atlanta. In 2010, he co-authored a more extensive, but still measured, analysis of the problem for the Proceedings of the National Academy of Sciences. The paper, titled "?'Control' laboratory rodents are metabolically morbid: Why it matters," laid out the case for how a rodent obesity epidemic might be affecting human health.
Standard lab rats and lab mice are insulin-resistant, hypertensive, and short-lived, he and his co-authors explained. Having unlimited access to food makes the animals prone to cancer, type-2 diabetes, and renal failure; it alters their gene expression in substantial ways; and it leads to cognitive decline. And there's reason to believe that ragged and rundown rodents will respond differently?abnormally, even?to experimental drugs.
Mattson has seen this problem in his own field of research. Twenty years ago, scientists started to develop some new ways to prevent brain damage after a stroke. A neurotransmitter called glutamate had been identified as a toxin for affected nerve cells, and a number of drug companies started working on ways to block its effects. The new medicines were tested in rats and mice with great success?but what worked in rodents failed in people. After a series of time-consuming and expensive clinical trials, the glutamate-blockers were declared a bust: They offered no benefit to human stroke patients.
Now Mattson has an idea for why the drugs didn't pan out: All the original test-animals were chubby. If there's something about the brain of an obese, sedentary rodent that amplifies the effects of a glutamate-blocker, that would explain why the drugs worked for a population of lab animals but not in the more diverse set of human patients. This past June, he published a paper confirming the hunch: When he put his test mice on a diet before administering the glutamate-blockers, the drugs' magical effects all but disappeared.
Many promising treatments could be failing for the same reason, Mattson argues, and other trials should be re-examined?but that's unlikely to happen anytime soon. "It comes down to money and resources," he says. "There's some fraction of studies that may have been compromised by [these] issues, but there's no way to know unless one does the experiment with the proper controls."
That's the drawback of the modern lab mouse. It's cheap, efficient, and highly standardized?all of which qualities have made it the favorite tool of large-scale biomedical research. But as Mattson points out, there's a danger to taking so much of our knowledge straight from the animal assembly line. The inbred, factory-farmed rodents in use today?raised by the millions in germ-free barrier rooms, overfed and understimulated and in some cases pumped through with antibiotics?may be placing unseen constraints on what we know and learn.
"This is important for scientists," says Mattson, "but they don't think about it at all."
Mattson is not the only one with doubts, nibbling away at the corner of his cage. The rise of the factory mouse has implications that extend far beyond his work on Parkinson's disease and stroke. By focusing so intently on one organism, raised in a certain way, we may be limiting our knowledge of cancer, too, and heart disease, and tuberculosis?the causes of death for many millions of people every year. If Mattson is right, science may be faced with a problem that is mind-boggling in its scope. Funding agencies in the United States and Europe will spend hundreds of millions of dollars in the coming years to further fiddle with and refine the standard organism, doubling down on a bet that goes back at least six decades: Establishing a single animal as the central determinant of how we study human illness, design new medicines, and learn about ourselves.
Just how ubiquitous is the experimental rodent? In the hierarchy of lab animal species, the rat and mouse rule as queen and king. A recent report from the European Union counted up the vertebrates used for experiments in 2008?that's every fish, bird, reptile, amphibian, and mammal that perished in a research setting, pretty much any animal more elaborate than a worm or fly?and found that fish and birds made up 15 percent; guinea pigs, rabbits, and hamsters contributed 5 percent; and horses, monkeys, pigs, and dogs added less than 1 percent. Taken together, lab rats and lab mice accounted for nearly all the rest?four-fifths of the 12 million animals used in total. If you extend those proportions around the world, the use of rodents is astonishing: Scientists are going through some 88 million rats and mice for their experiments and testing every year.
Dead mice pile up more than three times faster than dead rats, which makes sense given their relative size. Cheap, small animals tend to be killed in greater volumes than big ones. A researcher might run through several dozen mice, half a dozen rabbits, or a pair of monkeys to achieve the same result: one published paper. More striking, then, is the extent to which papers about rats and mice?however many animals go into each?dominate the academic literature. According to a recent survey of animal-use trends in neuroscience, almost half the journal pages published between 2000 and 2004 described experiments conducted on rats and mice.
A survey of the National Library of Medicine's database of more than 20 million academic citations shows the same trend across the whole of biomedicine. Since 1965, the number of published papers about dogs or cats has remained fairly constant. The same holds true for studies of guinea pigs and rabbits. But over that 44-year stretch, the number of papers involving mice and rats has more than quadrupled. What about the simpler organisms that researchers tend to poke and prod?yeast and zebra fish and fruit flies and roundworms?* By 2009, the mouse itself was responsible for three times as many papers as all of those combined.
That is to say, we've arrived at something like a monoculture in biomedicine. The great majority of how we understand disease, and attempt to cure it, derives from a couple of rodents, selected?for reasons that can seem somewhat arbitrary in retrospect?from all the thousands of other mammals, tens of thousands of other vertebrates, and millions of other animal species known to walk or swim or slither the Earth. We've taken the mouse and the rat out of their more natural habitats, from fields and barns and sewers, and refashioned them into the ultimate proxy for ourselves?a creature tailored to, and tailored by, the university basement and the corporate research park.
It's just the latest step in a trend that began more than a century ago. The splendid menagerie that once formed the basis for physiological study?the sheep, the raccoons, the pigeons, the frogs, the birds, the horses?has since the early 1900s been whittled down to a handful of key "model systems": Animals that are special for not being special, that happen to flourish under human care and whose genes we can manipulate most easily; the select and selected group that are supposed to stand in for all creation. Where scientists once tried to assemble knowledge from the splinters of nature, now they erect it from a few standardized parts: An assortment of mammals, some nematodes and fruit flies, E. coli bacteria and Saccharomyces yeast. Even this tiny toolkit of living things has in recent decades been shrunk down to a favored pair, the rat and mouse. The latter in particular has become a biological Swiss army knife?a handyman organism that can fix up data on cancer, diabetes, depression, post-traumatic stress, or any other disease, disorder, or inconvenience that could ever afflict a human being. The modern lab mouse is one of the most glorious products of industrial biomedicine. Yet this powerful tool might have reached the limit of its utility. What if it's taught us all it can?
The government's top researcher on tuberculosis?still one of the world's most deadly infections?seems to be running a midsized wildlife park out of his Maryland home. In a modest house on a tree-lined street in Germantown, Clif Barry keeps two kinds of turtles, three veiled chameleons, two Jackson's chameleons, six species of frogs, half a dozen fish tanks (filled with cichlids, goldfish, and piranhas, kept separately), two dogs (named Jacques and Gillian), and an Australian tree python. "I'm an animal person," he tells me. "My house would require a zookeeper's license if Montgomery County knew what I had."
 Clifton E. Barry, 3rd, National Institute of Allergy and Infectious Diseases
Clifton E. Barry, 3rd, National Institute of Allergy and Infectious DiseasesCourtesy of Clifton E. Barry, 3rd.
Twenty miles away in Bethesda, though, where Barry serves as chief of the Tuberculosis Research Section at the National Institute of Allergy and Infectious Diseases, a single animal has taken over the ecosystem. It has infested every paper and conference, and formed a living, writhing barrier to new drugs on their way to clinical trials. "We've always only tested things in mice," Barry tells me by phone one afternoon. "The truth is that for some questions, mice give you a very nice and easy model system for understanding what's happening in humans, but mice are mice, and people are people. If we look to the mouse to model every aspect of the disease for man, and to model cures, we're just wasting our time."
The problem, he says, begins with the three M?s. The process of drug discovery has been carried out in the same way for decades. You start by testing a new compound in a Petri dish, to find out whether it can slow the growth of a particular bacterium in culture. That gives you the smallest dose that has an effect, known as the minimum inhibitory concentration, or "MIC"?the first M. Then you move to a living animal: Does the compound have any effect on the course of disease in a lab mouse? If so, you've cleared the second M, and you're ready to test the compound in the third M, man. Each step leads to the next: No drug can be tested in man until it's been shown to work in mice, and no drug is tested in mice until it's been shown to have a reasonable effect in the dish. "The bad part of that," says Barry, "is that no part of it is predictive:" A new compound that succeeds in the dish might flunk out in the mouse, and something that can cure tuberculosis in a mouse could wash out in people.
Take the example of pyrazinamide, one of the front-line drugs in the treatment of tuberculosis. Along with three other antibiotics, it forms the cocktail that remains, despite ongoing research, our only way of defeating the infection. But pyrazinamide didn't make it through the three Ms: It does nothing in the dish?there's no MIC whatsoever?and it has a weak effect in mice. According to Barry, if a compound like that were discovered in 2011, it would never make its way into clinical trials. Forty years ago, the system wasn't so rigid. A prominent physician and researcher at Britain's Medical Research Council named Wallace Fox saw something intriguing in the animal data: Pyrazinamide's action seemed to persist when those of other drugs had stopped. He insisted on testing the drug in humans, and its effects were profound. The fact that nothing gets to humans today without first passing the mouse test, says Barry, "has cost us a new generation of medicines."
Indeed, there's been no real breakthrough in treating tuberculosis?no major pharmaceutical discoveries?since the early 1970s. The first antibiotic to have any success against the tuberculosis mycobacterium, the first that could penetrate its waxy coating, was discovered (and tested in guinea pigs) in the early 1940s. The best vaccine we have was first used in humans in 1921. (It works pretty well against severe childhood forms of the disease, but less so otherwise.) And the closest thing we have to a miracle cure?the multidrug cocktail that doesn?t work against every strain and requires a six-month course of treatment with severe side effects?was finalized during the Nixon administration. Since then, almost every new idea for how to treat TB has come from experiments on lab mice. These have given us enough new data to drown the infected in a tsunami of graphs and tables, to bury them in animal carcasses. Yet we've made little progress?OK, no progress at all?in treating the human disease. Tuberculosis causes more than 2 million deaths every year, and we're using the same medicines we had in 1972.
One major problem with the mouse model?and the source of its spotty track record in the clinic?is well-known among those in the field: The form of TB that mice happen to get isn't all that much like our own. A human case of the disease begins when infectious bacilli are inhaled into the lungs, where they grow in number as the immune system sends in its soldiers to fight them off. White blood cells swarm the bacteria in a rolling, alveolar scrum, forming a set of pearly-white masses the size of golf balls called granulomas. These are where the war between body and invader plays out in a series of contained skirmishes.
As more immune cells are recruited to fight off the infection, some of the balls swell and stratify into a more developed form: A sphere of macrophages and lymphocytes packed inside a fibrous shell, with a cottage cheese clump of dead cells and bacteria at its core. At this point the battle reaches a stalemate: The bacteria stop dividing; the body has controlled, but not eliminated, the infection. For most people who have the disease, it's a ceasefire that holds indefinitely.
But for some patients a latent case of tuberculosis can suddenly become active. The granulomas rupture and propagate, spilling thousands of organisms into the lungs, where they can be aerosolized, coughed up, and passed on to a new host. Left untreated, the infection migrates into the bloodstream and other organs; widespread inflammation leads to burst arteries or a ruptured esophagus; and in about half of all cases, the patient dies.
The layered granuloma is the defining feature of human tuberculosis: The place where the host fights the infection (successfully or not), and the necessary site of action for any drug. To cure the disease, a treatment must be able to penetrate each ball of cells, whatever its type or composition; every last bacterium must be destroyed. "It's the structure of those granulomas that makes it so difficult to treat TB," says Barry. And they simply don't exist in mice.
If you infect a mouse with TB?if you spritz a puff of infected air into its nostrils through a trumpet, as so many labs do around the world?the animal's lungs quickly fill up with bacteria and immune cells, like a nasty case of pneumonia. There are no discrete balls of tissue, no well-defined granulomas sheathed in fibrin, no array of structures that harbor the bugs at various stages of development. The mice have no special, latent form of TB, either, and no way to pass on the disease. They simply die, after a year or two, of a slow and progressive decline.
That's why we've made so little progress using mice to generate new drugs and treatments, Barry tells me. In the absence of a clear, granulomatous response upon which to model human disease, the second M has become a massive roadblock in the path to a cure. "The vast majority of the money that we spend in clinical trials based on mouse data is completely wasted," he says.
If you ask Clif Barry why we're still using the mouse to study tuberculosis, or Mark Mattson why we continue to test new drugs on obese and sedentary rodents, they'll tell you the same thing: Because that's what we've always done?we're in a rut. But to an outsider?say, a journalist who's trying to understand the place of the mouse in the broad enterprise of biomedicine?that explanation doesn't make sense. If you think of science as an industry of ideas, or a marketplace for medical technology, then there ought to be a clear and guiding incentive for greater efficiency in the lab?an invisible hand of self-interest with its fingers curled around every pipette and microtome. Any firm that finds a miracle drug can earn hundreds of millions in an IPO; any university professor who makes a breakthrough will gain fame and tenure; any disease that's cured may save countless lives. With so much on the line, how is it possible that all interested parties would be hamstrung by the same faulty method? Through what success or subterfuge did one particular rodent species earn its exalted spot among the three M?s of drug discovery? (Why should the second M stand for mouse, instead of monkey, marsupial, or mollusk?) In short: What's so wonderful about the lab mouse?
There's a standard answer to that question, one that can be repeated almost verbatim by biologists from across the spectrum of medical fields. The mouse is small, it's cheap, it's docile, and it's amenable to the most advanced tools of genetic engineering.
It's true that rats and mice are smaller, cheaper, and faster-breeding than many other mammals. Our last common ancestor lived 80 million years ago, which makes them closer cousins to us, in evolutionary terms, than either cats or dogs. (We share about 95 percent of our genome?although such comparisons tend to be more rhetorical than scientific.) And even among those who worry over the welfare of laboratory animals, there isn't much indignation at their use. (Rats and mice are denied many of the rights afforded other experimental mammals.) But a similar dossier could have been assembled in favor of any of a number of small creatures, some of which might be better-suited to answer certain questions. If you want to know about the visual system, for example, why use an animal that sniffs and whisks its way through the world? How about one that depends on its eyes, like a squirrel?
A rational calculation of its benefits and drawbacks misses the point of the modern lab animal. The real source of its influence, and the origin of its unique appeal to scientists around the world, is the simple fact of its having been chosen at all. Once we decided to focus our efforts on the rat and mouse, we learned how to fine-tune those species to fit our every need. When new technologies and methods entered the lab?improved products for animal care, better reagents for biochemistry, novel means for genetic manipulation?we tested them on, and tailored them to, our standard rodents. Meanwhile, the more rat and mouse data that accrued in scientific journals, the more tempting it was to devise follow-up experiments using the rat and mouse once more. In that sense, Barry and Mattson are right: We use these animals because that's what we've always done.
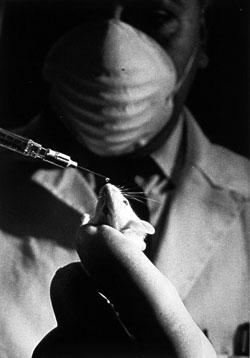 Mouse receives a drop of fluid from a syringe, U.S. Center for Biologics Evaluation and Research
Mouse receives a drop of fluid from a syringe, U.S. Center for Biologics Evaluation and ResearchPhotograph by Jerry Hecht, image from the National Library of Medicine.
The feedback loop began more than 60 years ago, when federal investment in biomedicine was growing at an exponential rate. To eradicate the last vestiges of infectious disease, win the war on cancer, and otherwise mobilize the nation's resources for an industrial revolution in science, the government needed a more streamlined research model?a lab animal, or a set of lab animals, that could be standardized and mass-produced in centralized facilities, and distributed across the country for use in all kinds of experiments. An efficient use of federal research funds demanded an efficient organism for research.
In part because of their size and breeding capacity, and in part because they'd been used in laboratories since the turn of the century, the rat and mouse were selected for this role. As major research grants began to flow from Washington in the 1950s and 1960s, private rodent breeders picked up huge contracts with government-funded labs. Animal factories expanded rapidly throughout the Northeast, and then around the world. Lab mice were shipped out to the front lines of industrial medicine for cancer-drug screenings, radiation exposure tests, and even routine medical procedures. (Some early pregnancy tests required the injection of urine into female mice.) Rats, meanwhile, were the favored test-animals for industrial toxicology, and the norm in studies of behavioral psychology. Supply and demand surged upward in a spiral of easy breeding and cheap slaughter.
Standardized cages, food pellets, and husbandry techniques lowered costs. (A basic lab mouse now runs about $5 and can be maintained for a nickel a day.) Standardized suppliers made research more convenient. (A scientist can order animals for second-day delivery online, or by calling 1-800-LAB-RATS.) And standardized breeds?which ensure that every mouse or rat is a virtual clone of both its siblings and its ancestors?made it easier to replicate and verify studies from one lab to another.
This process reached its apotheosis 30 years ago, when decades of investment in the rodent model paid off with an extraordinary invention: The transgenic mouse. In December 1980, a group at Yale announced that it had injected a bit of foreign DNA into a fertilized mouse egg, and then implanted the embryo to produce a transformed but healthy offspring. Now the mammalian genome could be modified at will.
One discovery came after another: In '81, researchers at Cambridge managed to grow a population of embryonic stem cells for the very first time?again derived from the standard lab mouse. By the end of the decade, these had made possible an even more powerful research tool, the "gene knockout" mouse. Now we could design living animals with precise twists or snips in the curls of their DNA. Individual genes could be mutated, deleted, or added de novo, and a golden age of mouse research soon followed. Over the next 20 years?and running up through the present day?lab animals were engineered such that genes could be switched on and off in specific parts of their bodies, or in response to drugs placed in their drinking water, or light flashed through fiber-optic cables. Not only did these tools provide a better understanding of the genes and molecules associated with disease, but they led to new approaches to research and treatment?those involving human stem cells, for example?that might one day make lab animals of any kind obsolete. The possibilities were endless. What started as a lab animal had become the biologist's most magnificent tinker toy.

Photograph from the World Health Organization.
As the power of the mouse model grew, researchers found fewer and fewer reasons to study anything else. Even the stalwart lab rat began to seem antiquated and unnecessary. (Genetic tools for the rat have been much slower to develop; its first embryonic stem cells were created in 2008, almost three decades after the same was accomplished in the mouse.) In the 1990s, the National Institutes of Health placed the transgenic lab mouse squarely at the vanguard of biomedical research?the very model of the modern model animal. Under the leadership of Harold Varmus, who won his Nobel Prize for using chicken and mouse cells to identify the genes most susceptible to cancer-causing mutation, the NIH announced that Mus musculus would be the second mammalian species to have its genome sequenced, after the human. "This animal is one of the most significant lab models for human disease," announced Varmus.
The government continued to support work on other organisms, as it always had. "Everybody would prefer to work with the simplest, cheapest system that can yield good results," Varmus explains by phone. "There's no doubt that NIH is very sensitive to the need to help provide some kind of support for developing [new] models." In 1997, he led an NIH-wide effort to promote the study of zebra fish, a kind of minnow that grows as a free-swimming, transparent embryo. Now it's considered one of the most important models for research in developmental biology and several other areas. Varmus is also excited about new ways to study cells taken from human tissue, healthy or diseased, and cultured in a dish. But when it came to the genome project, and the most significant investment in living animal models for biomedical research, the mouse was a clear favorite. "It was chosen because not only was there a rich history of work," he says, "but because we knew how to do two important things: We knew how to add genes to the mouse germ line, and we knew how to manipulate genes that were in the germ line."
The extraordinary tools available to mouse researchers?including the ability to create transgenic animals and then stash their sperm and eggs in freezers so they could be reproduced at will?made it possible to envision a full catalog of gene knockouts, what Varmus calls an "encyclopedic project" in biomedicine. "Everything you do with a mouse is an approximation," he says, but scientists have now created tens of thousands of kinds of mice, each one designed to answer its own set of experimental questions. "We use these models in the hopes that we can recapitulate human disease, and create a playing field for trying to prevent and treat disease."
Microbiologist JoAnne Flynn has some concerns about mice, an inkling that in some fields, at least, we may be too reliant on the standard breeds. But if you ask her why she's been using them in her lab to study TB for the last 20 years, she has no trouble rattling off an answer: "They don't cough. They don't spread the disease. They live in small cages. They're easy to infect by any route. ?"
I ask her to slow down so I can catch up in my notes.
 JoAnne Flynn, University of Pittsburgh School of Medicine
JoAnne Flynn, University of Pittsburgh School of MedicinePhotograph by by Joshua Franzos.
"? You can take out the lungs of four mice, and get very similar data from each one. You can knock out any gene in the mouse, not in a guinea pig or rabbit. You can turn a gene on for a little while and then turn it off. You have every immunological reagent?all kinds of assays and antibodies. ?"
In her biosafety-level-3 laboratory at the University of Pittsburgh, Flynn has used the standard animal model of TB to work out how particular elements of the immune system fight off the disease. In 1995, for instance, she applied knockout genes and monoclonal antibodies?two methods that were developed in the mouse?to show that a signaling protein called TNF-alpha helps mice survive an infection for months, instead of weeks.
"? You can have mice of different genetic backgrounds, and determine which genes make a difference. You can do vaccine studies. You can have cells that turn certain colors. You can make T-cells turn yellow when they do something. You can do trafficking studies, inject cells and watch where they go. You can collaborate with people to make new kinds of mice. ?"
When she learned that doctors might prescribe TNF-inhibitors to patients with autoimmune diseases like rheumatoid arthritis, Flynn got in touch with one of the pharmaceutical companies making the drugs. If the mouse studies were right, she argued, then the treatment could be dangerous?especially for patients who were carrying latent TB.
In 2001, researchers at Boston University and at the Food and Drug Administration sifted through the database of adverse event reports for a popular TNF-inhibitor, and found more than five dozen cases of tuberculosis. The rate of infection among people taking the drug turned out to be four times higher than it was for other people of a similar health status. Twelve patients had died from the disease. Flynn was right. The results were published in the New England Journal of Medicine.
"? It may not be everything you want, but the mouse is a really flexible model," she says, finally taking a breath. Among her colleagues, the ratio of studies performed on mice to those using any other species of lab animal is about 50-to-1. "A mouse is an extremely reductive model, and it allows you to ask very specific questions," she continues. The role of TNF-alpha is just one of its many contributions to the study of tuberculosis: "The progress in TB research has been astronomical. But there has been a reckoning. People have had to stop and think, 'We're doing something wrong. We're not finding drug targets. We're not finding drugs that work the way we think they're going to work.'?"
For Flynn, that reckoning came 10 years ago. She'd made a successful career working out the details of tuberculosis immunology in the mouse. But she knew that an effective, short-term treatment for latent TB?the form of the disease that affects roughly one-third of the world's population?wasn't going to emerge from studies like hers. The lab mice that she'd been using since her first job as a post-doc, and that nearly every other tuberculosis lab in the world had been using for decades, can only get an active infection. "That's a huge downfall of the mouse," she says. "I had to ask myself, am I missing something?"
If she wanted to study latent TB, she'd have to switch over to a new lab animal?the crab-eating macaque. Monkeys contract tuberculosis as humans do: Their lungs fill up with granulomas of different types and structures, and in 60 percent of cases they sustain the infection with no symptoms whatsoever. Converting her lab would be a daunting project, though. Monkeys are many thousands of times more expensive to keep around than mice, especially in the controlled environments used for the study of infectious disease. It takes longer to finish monkey experiments and publish monkey papers, which slows down the research as well as the process of getting grants and otherwise advancing your career. There's also the problem of small sample sizes, and the lack of genetic tools and reagents. Flynn went ahead with her plan nonetheless, and even after a decade's worth of monkey experiments she still spends some of her time rehashing facts about TB?such as the role of CD4 immune cells in containing the infection?that she'd established in the mouse years ago. These are data that many of her colleagues would have accepted without question. "It's incredibly risky and difficult for us," she says. "We took a leap."
Abandoning the mouse model may not be worth the time and money required, says Richard Chaisson, director of the Center for TB Research at Johns Hopkins University. "There are always questions about whether new drugs will behave in the mouse in the same way old drugs do, but so far nothing has come close to the accuracy of the mouse model for telling us what to expect in people," he explains in an email. "There is a great deal of interest in primate models right now, but there is no current evidence that this very expensive approach is as good as, let alone better than, the mouse."
Such evidence would be hard to come by. It's not clear how one might prove, in a satisfying and scientific way, that any given lab animal is better than another. We can't go back and spend the last 50 years studying monkeys instead of mice, and then count how many new drugs came as a result. The history of biomedicine runs in one direction only: There are no statistics to compare; it's an experiment that can't be repeated.
Before the mouse started to take over the field in the 1960s, the classical models for TB research were rabbits and guinea pigs?small, cheap mammals with a granulomatous response that's not too far off from what happens in humans. Since then, a number of new models have emerged: Goldfish and frogs develop a very similar disease, and so can the zebra fish, the minnow championed by the NIH: Its granulomas can be seen forming in real time. Flynn sees a place in the field for every one of these models. Different questions require different methods, she says. But if you want to study the course of latent TB in an animal host, than you're stuck with macaques or another nonhuman primate.
As for mice, "it's not that [they] aren't useful," she says, "or even that they're not the most useful possible system. Rather, it's that by focusing only on the mouse, we're running a grave risk."
For a scientist, switching lab animals in the middle of your career is something like changing religions. The academic world tends to cluster by model system: "Mouse people" talk to mouse people; "monkey people" talk to monkey people; each group exists within its own fibrous granuloma of meetings, conferences and review committees. When you submit a grant application to the NIH, or a manuscript to a scientific journal, the peers who assess your work are likely to be fellow travelers, members of your animal clique. Changing over from one species to another means backing out of a social sphere, and when the sphere you're leaving happens to be the biggest and best-funded, well: "I used to lose sleep over this," Flynn tells me at one point. And then later, with a chuckle: "I always say it's career suicide."
Clif Barry, the man of the Germantown menagerie, seems like he knows the value of standardization. When I meet him in his office at the National Institutes of Health in July, he's wearing a pair of black pants, a black belt, and black socks; a tight-fitting black polo shirt and a pair of black, rectangular eyeglasses from Armani Exchange; there's a pair of black sneakers beside him on the floor. The monochrome wardrobe makes life easier, he tells me. Everything matches everything else. It's one less thing to worry about.
Yet Barry has moved away from the standard model for tuberculosis research?the one that can be ordered by phone, hundreds at a time, in well-characterized strains and breeds. Like JoAnne Flynn, he's rejected the animal that allows for the most intricate molecular manipulations, and the most rapid screenings of new drugs. For him, the vaunted efficiencies of the murine-industrial complex are an illusion. Drugs go from mice to man, and then they fail.
 A marmoset at the National Institute of Allergy and Infectious Diseases
A marmoset at the National Institute of Allergy and Infectious DiseasesCourtesy of the Tuberculosis Research Section, National Institute of Allergy and Infectious Diseases.
In his own lab, he's spent the last three years working out what he calls an "advanced model" for drug-discovery?a brown-eyed primate with tufts of white poking from above its ears, and mottled, black-and-gray fur. At 7 inches in length, and weighing just half a pound, a marmoset isn't any bigger than a lab rat, and it reaches sexual maturity in half the time it takes a macaque. Around 90 percent of all marmoset births result in chimeric siblings with matching immune systems, which give Barry an easy way to control each experiment: It's like he's running a series of tiny twin studies. One marmoset gets a new drug and its littermate doesn't; then he compares the results. There's even hope that the marmoset might be susceptible to wholesale genetic engineering: In 2009, a Japanese team created a transgenic line of marmosets?the first time that had ever been accomplished in a primate species.
Compared with mouse research, though, Barry's advanced model moves at a glacial pace. His animals need 35 times more space than mice, and round-the-clock veterinary care. They're contagious, so lab workers have to wear puffy biohazard suits with built-in fans. And each animal must be trained laboriously to accept drug injections and crawl into a CT scanner. In all, says Barry, the switch to marmosets has reduced the number of compounds he can test in the lab by a factor of 50 or 100. "I'm continuously second-guessing it," he admits. Could a slower, less efficient animal model really help us cure tuberculosis?
Barry is betting that it can, but there's an eminence grise standing against him: 82-year-old doctor and microbiologist Jacques Grosset. A Frenchman with fluffy, white hair, Grosset has been training the next generation of TB researchers for the last half-century, screening new drugs and combination therapies in a strain of inbred mouse called the Bagg's Albino. They infect and inject hundreds of them at a time, in an ongoing parade of pale ears, pink eyes, and matching snow-white coats.
In the 1960s and ?70s, Grosset participated in the development of the classic, four-drug treatment for TB; since then, he's run his mice through various tweaks and substitutions that might improve the therapy. Some of these have turned out more useful than others. In 1989, he observed that one of the ingredients in the cocktail seemed to be spoiling the mix: Infected lab mice were better off when he removed it altogether. Fifteen years later, Grosset's team made another big discovery: When they replaced the troublesome drug with a new one called moxifloxacin, their mice were cured in record time. "The findings suggest that this regimen has the potential to substantially shorten the duration of therapy needed to cure human tuberculosis," they wrote.
The video above shows lung scans from twin marmosets with tuberculosis. The sibling on the left suffers from a more virulent strain of the disease known as the "Beijing clade," which may be especially dangerous to humans. The distinction is invisible in mouse models.
Barry considers Grosset a friend?he even named one of his frizzy-haired bichon frises Jacques in his honor?but he was skeptical when the moxifloxacin finding was announced at a lung-disease conference in Paris. Everyone was talking about how to get Grosset's new regimen into the clinic as quickly as possible, but there wasn't much reason to think the mouse data would translate to humans. "People were going wild; it was a frenzy. And I stood up at the microphone and said, 'This is a mistake. It's going to fail. And when it fails, I don't want to hear anything more about the mouse model.'?"
No one listened. A major trial soon followed, enrolling more than 400 patients from 26 hospitals on four continents?the kind of research that typically runs tens of millions of dollars?and, sure enough, the results were disastrous. Swapping in the new drug had no observable effect on the course of treatment. When the scientists in charge, Grosset among them, published their report in 2009, they could only scratch their heads over "the apparent discordance between murine results and results of this clinical study."
A second group ran a very similar, and similarly expensive, clinical trial shortly thereafter, this time focusing on TB in American and Asian patients, rather than the Black Africans who predominated in Grosset's study. The results were more or less the same. "We keep getting led down the garden path," Barry argues. The doctors who devised the classic treatment 40 years ago didn't need detailed mouse data?they found their cure with a methodical, brute-force approach: a series of human trials that spanned the better part of two decades and tested every possible combination of exposures. "The way those four drugs were put together is incredible. It's never to be seen again."
Since that happened, we've had thousands of mouse studies of tuberculosis, yet not one of them has ever been used to pick a new drug regimen that succeeded in clinical trials. "This isn't just true for TB; it's true for virtually every disease," he tells me. "We're spending more and more money and we're not getting more and more drug candidates."
Clif Barry is not the only scientist frustrated by the pace of progress. It's not at all clear that the rise of the mouse?and the million research papers that resulted from it?has produced a revolution in public health.
It's hard to measure such things in aggregate, of course, but science and health policymakers have reached an uneasy consensus on this fact: We're at a moment of crisis in drug discovery. Last winter, current NIH director Francis Collins established a new institute (his agency's 28th) to address the "pipeline problem" in biomedicine: Despite pouring billions of dollars into research every year, our rate of innovation has slowed to a trickle. It takes more than a decade, and some $800 million, to produce a viable, new drug; among the compounds considered for testing, only 1 in 10,000 come to fruition.
These are grim statistics, the kind that get bandied about in discussions of health care reform and the national budget deficit. We've seen a revolution in molecular genetics, yet medical research has been spinning down to a halt. The United States spends more than twice as much on the health of its citizens as do most Western nations, yet ranks middling to poor on life expectancy, infant mortality, cancer survival rates, and many other important measures. No one knows why.
Some worry that with all our fine-tuned genetic methods, we've gotten hung up on the small-scale workings of disease. It's missing the forest for the trees, goes the argument: Doctors did better in the 1970s, when they tried to find drugs that worked in the clinic, regardless of the mechanism. Others acquit the basic science, but say our clinical trials?the human tests?are run so poorly that good drugs, safe and effective ones, are flunking out. Another group wonders if we're getting held up at the preclinical stage: Researchers may be using shoddy statistical methods in their animal work, or scrapping negative results, or even adding subtle bias to their data when they pluck certain mice from the cage instead of others.
Here's another way to explain the heavy expense and slow rate of return in biomedicine: Maybe the animals themselves are causing the problem. Assembly-line rats and mice have become the standard vehicles of basic research and preclinical testing across the spectrum of disease. It's a one-size-fits-all approach to science. What if that one size were way too big?
For some maladies, like tuberculosis, the rodent model may be inadequate to its core, lacking in the basic mechanisms of human disease. For others, the mere fact of the rodents' ubiquity?and the standard ways in which they're used?could be hindering research. Jeffrey Mogil, who studies chronic pain at McGill University in Montreal, points out that almost every datum of mouse research comes from male specimens, despite the fact that male and female mice respond to pain in different ways. Mark Mattson laments the factory breeding conditions and mass-husbandry practices that bias his experiments and leave his control animals overfed and unwell.
Perhaps the researchers have come to resemble their favored species: So complacent and sedentary in their methods, so well-fed on government grants, that any flaws in the model have gone unnoticed, sliding by like wonky widgets on a conveyor belt. It could be that the investigators and the investigated are locked together in the mindless machinery of science, joined hand and paw in the manufacture of knowledge. If there were something wrong with the rodent?not just its body weight or exercise habits, but in its fundamental utility as an instrument of learning?the scientists may not realize it at all.
It might take an outsider to see the problem, then, someone standing beyond the factory gates. Starting in the early 1990s, and coincident with the rise of the transgenic mouse, a set of historians and philosophers of science began to construct a formal critique of industrial biomedicine. They acknowledge, first of all, that the mouse and rat have been enormously productive, and that standardization brought with it unparalleled economies of scale. But they wonder whether a laboratory monoculture, with such a glut of cheap data, can be truly sustainable. Rats and mice were never so good at curing disease as they were at making data for its own sake: We have a million papers' worth already, and could soon have a million more: experimental results that might one day be mothballed in dusty stacks, next to those of some other research juggernauts?phrenology, miasmatism, radical behaviorism?that rolled along for decades before coming to a creaking halt, not so useful and not much cited.
Whether the critics are paranoids or prophets, the hordes of mice are marching on. Transgenic models have colonized the whole of biomedicine, and their influence grows daily. At a meeting in London last year, scientists announced the inevitable next step?an audacious plan to mutate every single gene in the murine genome and record the function of each one in a public database. It?s a massive, multigovernment undertaking that would reinforce the mouse's position as the most thoroughly examined and explicated higher organism on the planet??a historic opportunity,? as one of its organizers, Mark Moore, describes it, ?to systematically learn everything about a mammal for the first time.?
The project, which has the support of science agencies in the United States and Europe, will cost at least $900 million. That is to say it?s a monumental piece of megascience with a price tag on the order of the Large Hadron Collider. In the end, we?ll have an archive of stem cell lines?a community warehouse from which researchers can create (and order off the Web) an animal with any one of its 20,000 genes inactivated. And each of those myriad versions of the mouse will have been run through a standard battery of tests and had its essential features stored in a public database.
A bio-utopian dream like this can imprison us with grand expectations. Once you've invested so much into a single model, how do you know when that model's utility has run its course and it's time to move on? Vinny Lynch, an evolutionary biologist at Yale University, has a quixotic vision for the future of biology. It's the "hourglass model" of animal research: We started out in what was almost the random study of nature?a broad biology of birds and snails and elephants, uninformed by deeper understanding. Then came the narrowing of the 20th century, when all our research efforts were funneled through a slender sieve of model systems. Now we've reached the limit of that bottleneck?the inherent limitations of the mouse, let's say?and it's time to broaden out once more, to apply all the knowledge we've gained over the last hundred years to selecting new animals and new systems, and broaden out into a more rational, relevant science.
In a paper titled "Use with caution: Developmental systems divergence and potential pitfalls of animal models," Lynch makes the argument for entering this next phase. He tells me we may already have reached a tipping point, in fact. The mouse monopoly is teetering in the face of cheaper, faster genetic technologies. More and more species are having their genomes sequenced and their DNA manipulated in the lab. In a hamlet on the north shore of Long Island, the Cold Spring Harbor Laboratory keeps track of where we might be headed. Its Website declares, "the variety of organisms studied is currently undergoing a massive expansion." Among the emerging models profiled on the site are the wallaby and the wasp, the quail and the snail, the yam and the snapdragon.
These are encouraging indicators. They suggest that, in spite of the billion-dollar mouse mutation project now on the horizon, we may soon find ourselves backing away from the industry standards that have dominated biomedical research for so many decades. Clif Barry and JoAnne Flynn may be at the vanguard of a new movement within science, a retreat from the seductions of model organism-ism to something more diverse?a throwback, perhaps, to the slower, more comparative style of the 19th century, when theories were constructed from the differences among the many, rather than the similarities of a few.
Every standard organism has its limit, some finite store of knowledge that can be gleaned from it. There will come a point when we?ll have learned so much about the workings of a mouse, and dissected its every organ in such nanoscopic detail, that its veins of data run dry. There's a depth at which the science begins to founder, where its explorations become so profound and so specific, so lost in the letters of a foreign genome, that its reports to the academy no longer mean anything at all. To move beyond this dead end, to develop new treatments for disease and understandings of how it works, we'll need a more inclusive science, a more organic science, a research enterprise with room for mice and flies and worms, but marmosets, too?all the bugs and birds and beasts that live together in a vibrant ecosystem of discovery.
More from this series: The one mouse who rules over all other mice, and the invincible naked mole-rat. Also, a history of rodent mazes. Read the text-only version of all three parts here.
Correction, Nov. 19, 2011: The article originally implied that C. elegans is an earthworm, not a roundworm. (Return to the corrected sentence.)
Source: http://feeds.slate.com/click.phdo?i=d70dbf5316d175c15e0db7d455fddb9b
bonjovi dead sam shepard sam shepard johnny knox johnny knox bonjovi bonjovi
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.